
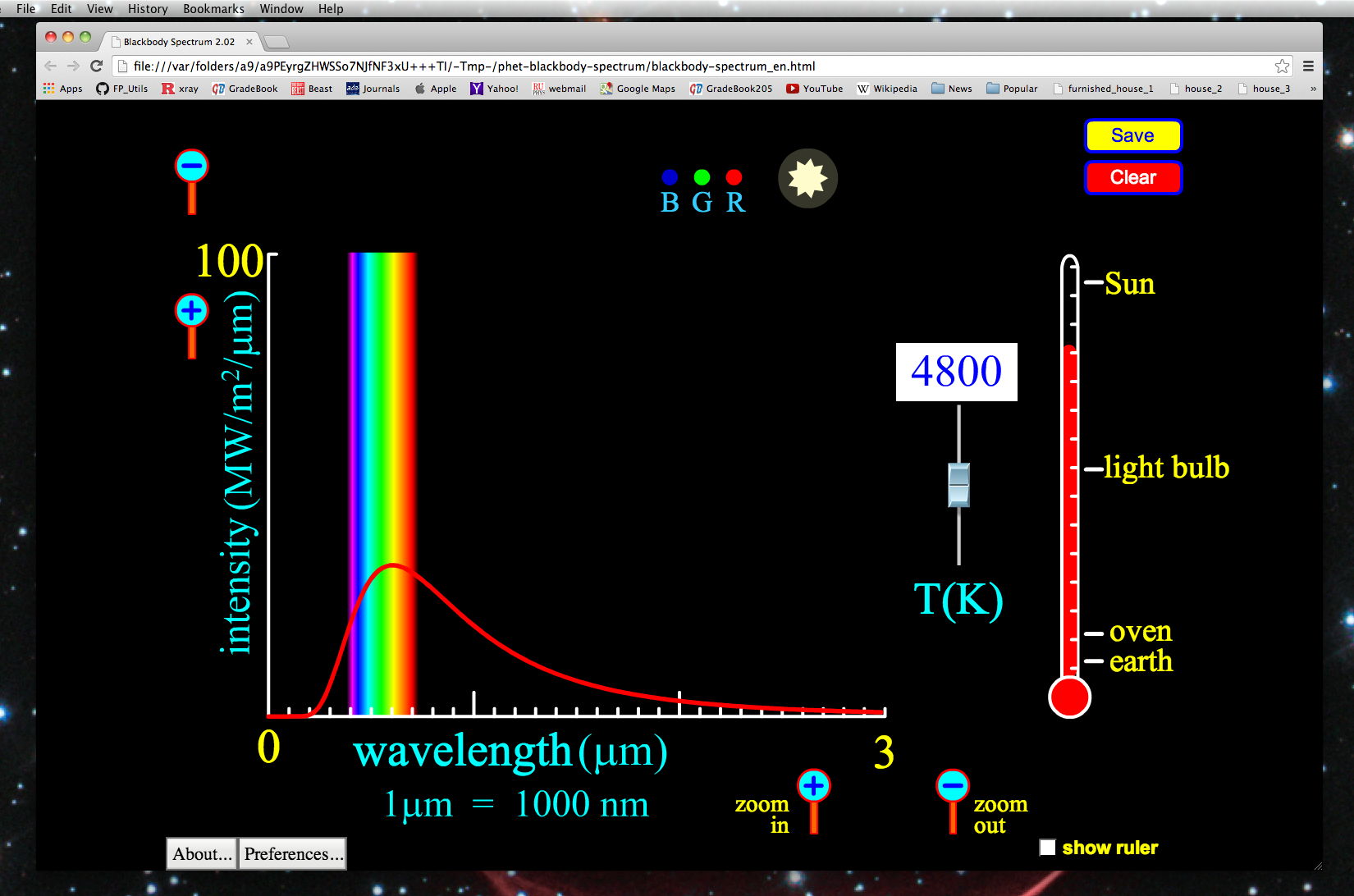
#Atomic emission spectra series
The effluent from the column is directed on to a moving cold window where the peaks in the chromatogram can be condensed as a series of spots about 100 μm across. For higher sensitivity a more elaborate deposition method is available. This achieves detection limits of ∼10 ng for strongly absorbing compounds. The usual approach is to pass the gas flow from the GC column through a light pipe ∼1 mm in diameter and 10 cm long. To maximize sensitivity, the sample has to be confined to a small area. The most important of these is gas chromatography. Separation techniques are needed in order to identify individual components in mixtures. The most sophisticated version of this method is to couple a gas cell in the spectrometer to a thermogravimetric analyser and measure the products evolved at different temperatures. There are collections of the spectra of decomposition products from polymers. Commercial devices also allow gaseous products to be collected. The simplest approach is to heat the sample in the bottom of a long test-tube so that decomposition products condense at the top of the tube and can be measured by the usual methods. Pyrolysis can provide qualitative information from intractable samples without requiring expensive accessories. Emission is very useful to obtain spectra from thin coatings on uneven metal surfaces when diffuse reflection measurements would require a small sample. The reflection spectra are usually measured more easily than emission. If the sample is opaque, the information provided is identical with that in the reflection spectrum since the sum of transmission, reflection and emission at any wavelength adds up to one. Spragg, in Encyclopedia of Spectroscopy and Spectrometry (Second Edition), 1999 Miscellaneous TechniquesĮmission spectra can be obtained without any sample preparation, but they are of very limited use. In some cases the absorption bands are so narrow as to give the appearance of a line absorption spectrum. Identification of these groupings is therefore possible by examining absorption spectra of substances of unknown, or uncertain, composition or molecular structure. Dark bands appear in the spectrum of the transmitted light, representing missing wavelengths due to absorption, characteristic of the particular molecular groupings present in the substance through which the light is passing. These bands are found to consist of very many closely spaced lines, the spacing between the lines decreasing towards the sharp end of the band (or “band head”).īand absorption spectra are produced by inorganic compounds in solution and, in general, by organic compounds. This gives a “fluted” appearance to the spectrum, which is called a band spectrum. The luminous regions have a sharp “cut-off” at one end but are diffuse at the other. Courses, 1970 Band spectraĮmission spectra of chemical compounds, or of substances containing atoms grouped together to form molecules, may produce emission spectra in which regions of the spectrum appear, separated by dark spaces. For highly ionized atoms, the lines are found in the extreme UV or x-ray region.Īs the relative intensity of the lines in an atomic spectrum varies with temperature, analysis of the lines in the spectrum of a star (say) can give an estimate of the temperature of the star’s surface (photosphere).R.A. The light electronic transitions in atoms produces may not be in the visual part of the electromagnetic spectrum, but for atoms that are neutral or have lost only one or two electrons (yes, ‘atomic spectra’ refers to the line spectrum of ions too!), most lines are in the UV, visual, or near infrared.
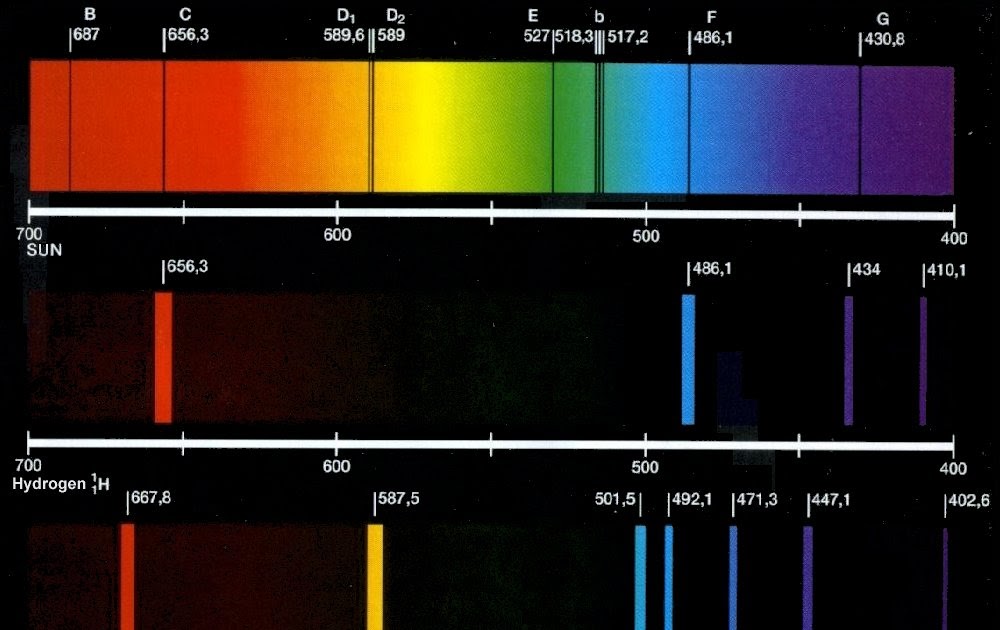
Of course, for an extragalactic object – a quasar, perhaps – you need more than one line to make a certain identification … because the universe is expanding (and so you don’t know how much just one line may have been redshifted). As the atomic electron energy levels are unique to each element, the lines in a spectrum (emission or absorption) can be used to identify the elements present in the source (a star, say) or gas between the source and us (e.g.


 0 kommentar(er)
0 kommentar(er)
